Britain’s approval of antiviral pill to fight COVID is greeted as good news; but no approval granted yet for Caribbean use
Worldwide, people are expressing excitement over the approval of an anti-viral pill that will be deployed in the fight against COVID-19.
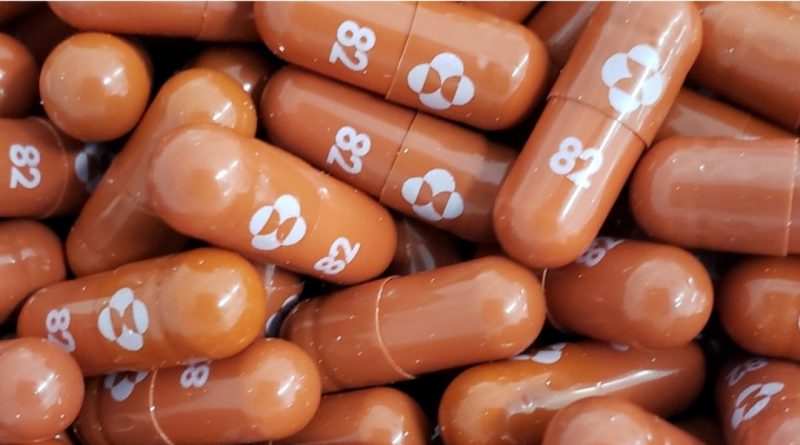
With this groundbreaking move, Britain becomes the first country to grant conditional authorization of the Merck drug, Molnupiravir (Mol-new-pee-raveer).
Reportedly, the tablet works by blocking the ability of the coronavirus to reproduce. Therefore, manufacturers claim the pill will cut hospitalizations and COVID-19 deaths by half.
However, the pill is still awaiting the approval of the US Food and Drug Administration and the Caribbean Public Health Agency.
Once the requisite approval is granted by the World Health Organisation, there will be a rollout of the drug within the region, reports say.
Prime Minister Gaston Browne had indicated, some weeks ago, that once the new pill is available the Government will seek to obtain it.
Some residents have said they would prefer to take the pill, rather than a vaccine, since the manufacturers have claimed the tablet has fewer side effects. Those who are terrified of needles also welcomed the news. Should persons decide to stop taking the vaccines once the pill is available, a local man wants to know whether this will leave the Government holding stocks of the current drugs.